If your job requires you to measure acidity, then it is helpful for you to understand what this thing “acidity” actually is, accepting that not everyone is a chemist. We talk about acidity and how a ph measurement instrument works today.

What Is This Thing Called Acidity?
Acidity and alkalinity are forms of chemicals that are water-soluble. Free Hydrogen Ions do not exist in water. This happens when an Acid is introduced. Acid forms positive hydrogen ions as the H+ ions are released from the acid and bond with the water. Alkalines (or bases) are chemical compounds that attract Hydrogen Ions, resulting in a solution of negative hydroxide ions, also known as Hydroxyl Ions (-OH). Strong acids (more positive hydrogen bonds) and strong alkali (more negative hydrogen bonds) solutions can both burn you.
How Does A pH Measurement Instrument Measure Hydrogen Ion Concentration?
Acidic solutions present a denser concentration of positive hydrogen ions and can therefore potentially create an electric current in the right conditions.
A pH measurement instrument uses this to measure voltage and compares it to a control substance with a known voltage. The difference in the two measurements is the pH.
What Is A pH Measurement System Usually Made Of?
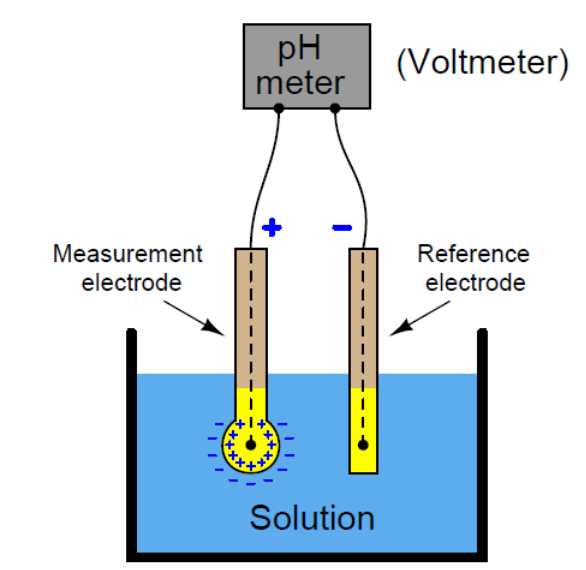
There are primarily two main components within the instrument.
Firstly, something needs to be placed in the solution being measured (ie. a probe or electrode), and secondly, a reading needs to be taken by a transmitter.
Each probe contains 2 electrodes; the measuring glass electrode and the reference electrode, which creates a closed electrical circuit.
The glass electrode has a silver-based wire hanging in potassium chloride solution within a thin capsule of specialised glass. The reference electrode has a potassium chloride wire in the same solution.
Potassium chloride has a pH of 7. If the solution being measured, for example, is more acidic than pH7, the glass electrode measures the different voltages of the test solution versus the pH7.
How Does the Measurement Work?
As the glass electrode comes into contact with the test solution, some hydrogen ions of the test solution connect with the glass electrode outer surface and swap places with the metal ions within it. This ion-swapping also occurs on the inside surface of the thin glass capsule.
The glass capsule now has different acidity on either side of its glass, which creates different electrical charges and a tiny voltage difference between the silver electrode and the potassium chloride. The digital display shows the pH interpretation of this.
The greater the hydrogen ion activity between the inside surface to the outside surface, the bigger the difference in voltage and the lower (more acidic) the indicated pH value will be.
To learn more, take a look at the following brochure https://bdih-prod-assetcentralapi-assetcentral-rest-srv.cfapps.eu10.hana.ondemand.com/files/DLA/005056A500261EDA9AC31DEAE35A6803/CP00010CEN_1520_pH-selection-guide20.pdf
We’ve been measuring New Zealand for fifty years! Contact us to get your quality pH measurement instrument today.
